What is pH?
pH is a measurement of the acidity or alkalinity of a solution. It provides a value on a scale from 0 to 14 where 7 is neutral, less than 7 is acidic, and greater than 7 is alkaline (or basic).
The closer you move towards 0, the more a solution is acidic, and the closer you move to 14, the more a solution is alkaline.
pH is often depicted on a graphical colour scale as shown below:
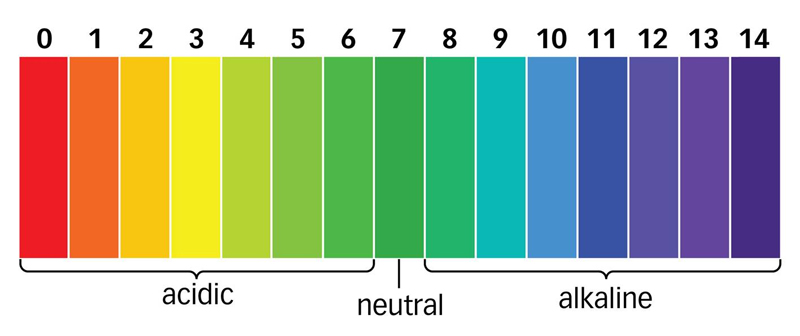
When talking about water, its pH value is related directly to the ratio of positively charged hydrogen ions [H+] and negatively charged hydroxyl ions [OH-].
When water has an equal concentration of H+ ions and OH- ions, it is said to be neutral (pH=7)
When water has a greater concentration of H+ ions, it is said to be acidic (pH<7) When a solution has a greater concentration of OH-, it is said to be alkaline (pH>7).
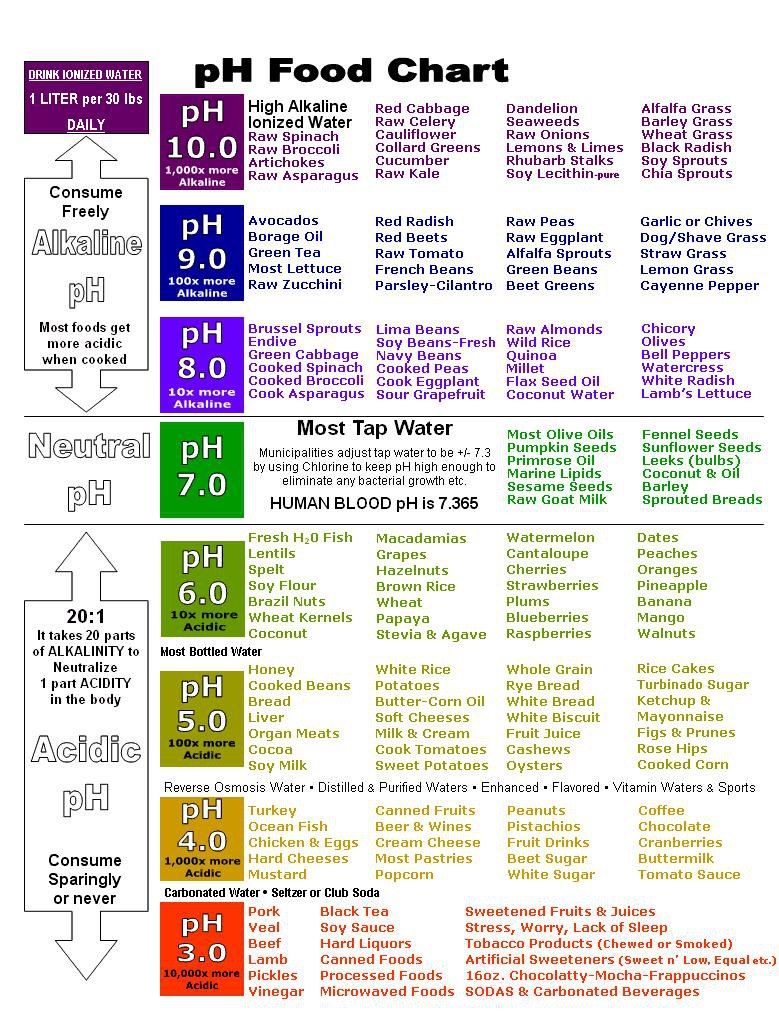
Disclaimer:
These information are used for learning, if there is any infringement, please contact us to delete.
Information and statements made on this web are for education purposes and are not intended to replace the advice of your family doctor.
The products mentioned here are not intended to diagnose, treat, cure or prevent any disease or health condition.No medical claims are being made.
